Vinyl Alcohol Tautomerization
Having the same number of each atom but with a.
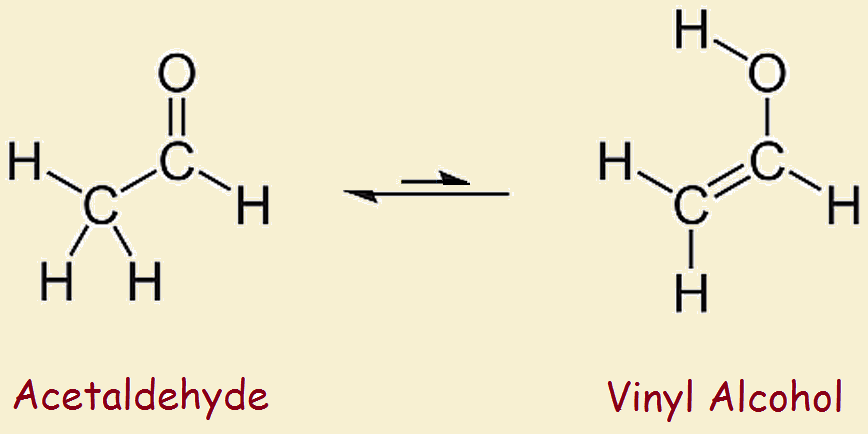
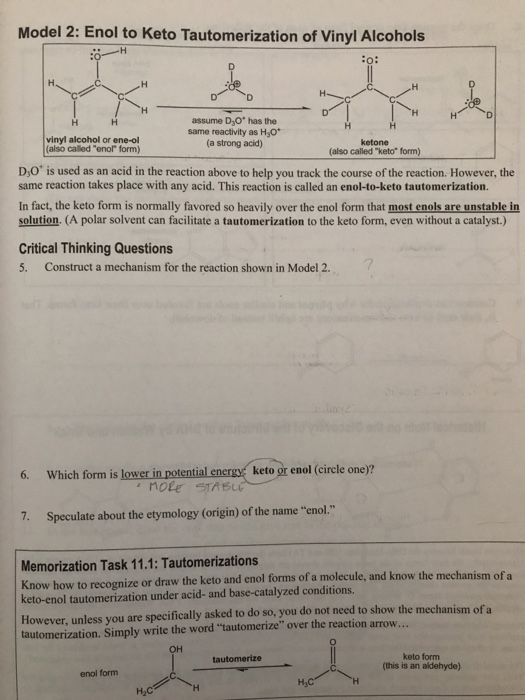
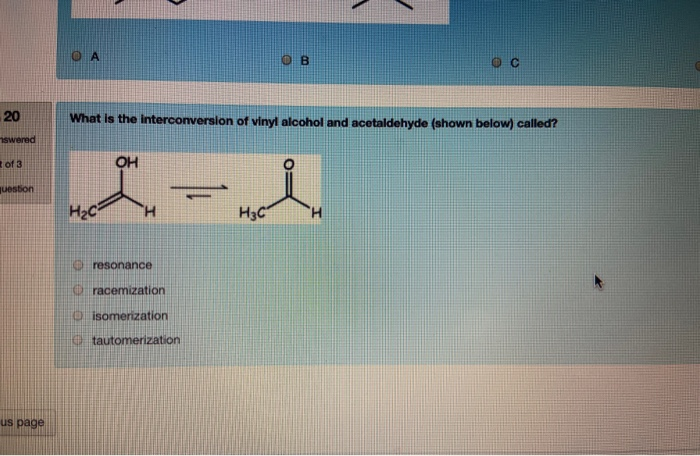
Vinyl alcohol tautomerization. This reaction is shown below in fig 1. 1 tautomerization of vinyl alcohol to acetaldehyde. The keto part refers to the carbonyl group typical of ketones and aldehydes c o. Determines benchmark reaction barrier heights for the catalyzed tautomerization.
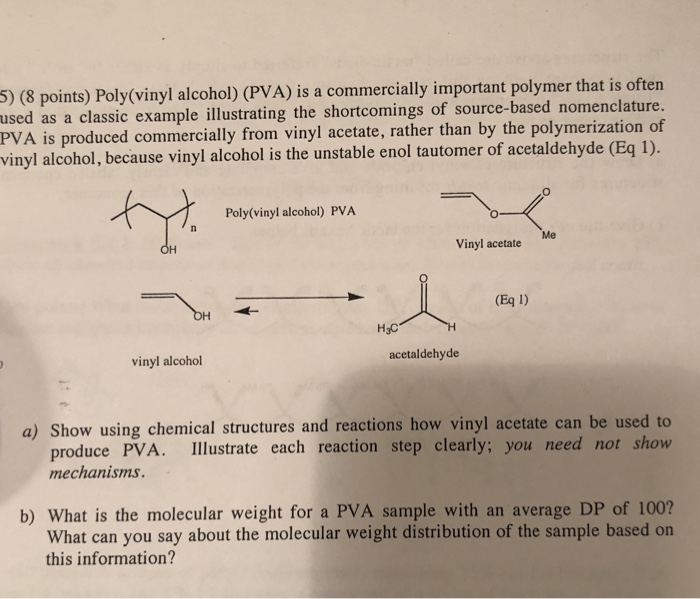
Tautomers are constitutional isomers. In this post we wish to determine the reaction energy barrier and transition state structure for the tautomerization reaction of vinyl alcohol converting to acetaldehyde using dft. Vinyl alcohol rearranges slightly under ordinary conditions. Tautomerization of vinyl alcohol.
The enol part refers to the two functionalities ene and ol. The tautomerization can also be catalyzed via photochemical process. Vinyl alcohol can be formed by the pyrolytic elimination of water from ethylene glycol at a temperature of 900 c and low pressure. Inorganic acids can efficiently catalyze the tautomerization reaction.
It tautomerizes to the more stable species acetaldehyde. When collisions at atmospheric pressure are included the model quantitatively reproduces previously reported quantum yields for photodissociation at all pressures and. Under normal conditions vinyl alcohol converts tautomerizes to acetaldehyde. H 2 so 4 and hclo 4 catalysts lead to near zero reaction barrier heights.
The model quantitatively reproduces the experiments and shows unequivocally that keto enol photo tautomerization forming vinyl alcohol ethenol is the crucial first step.