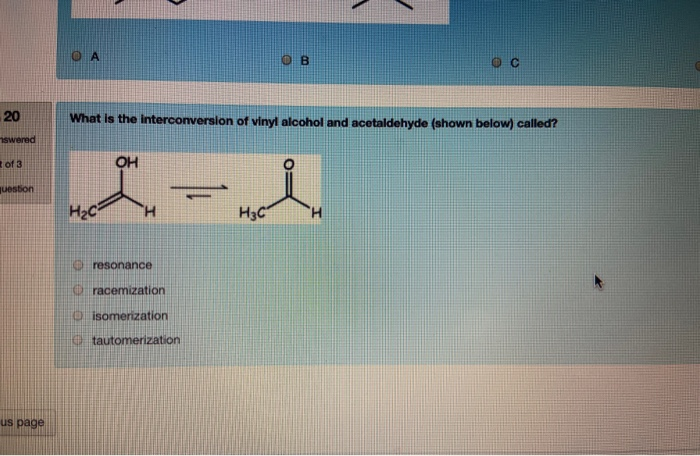
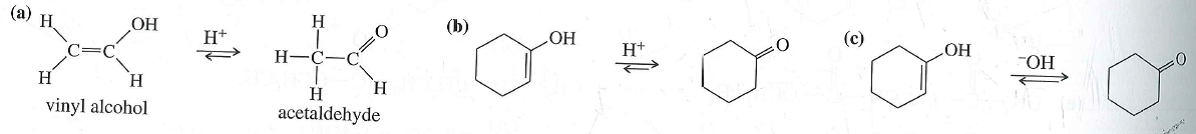
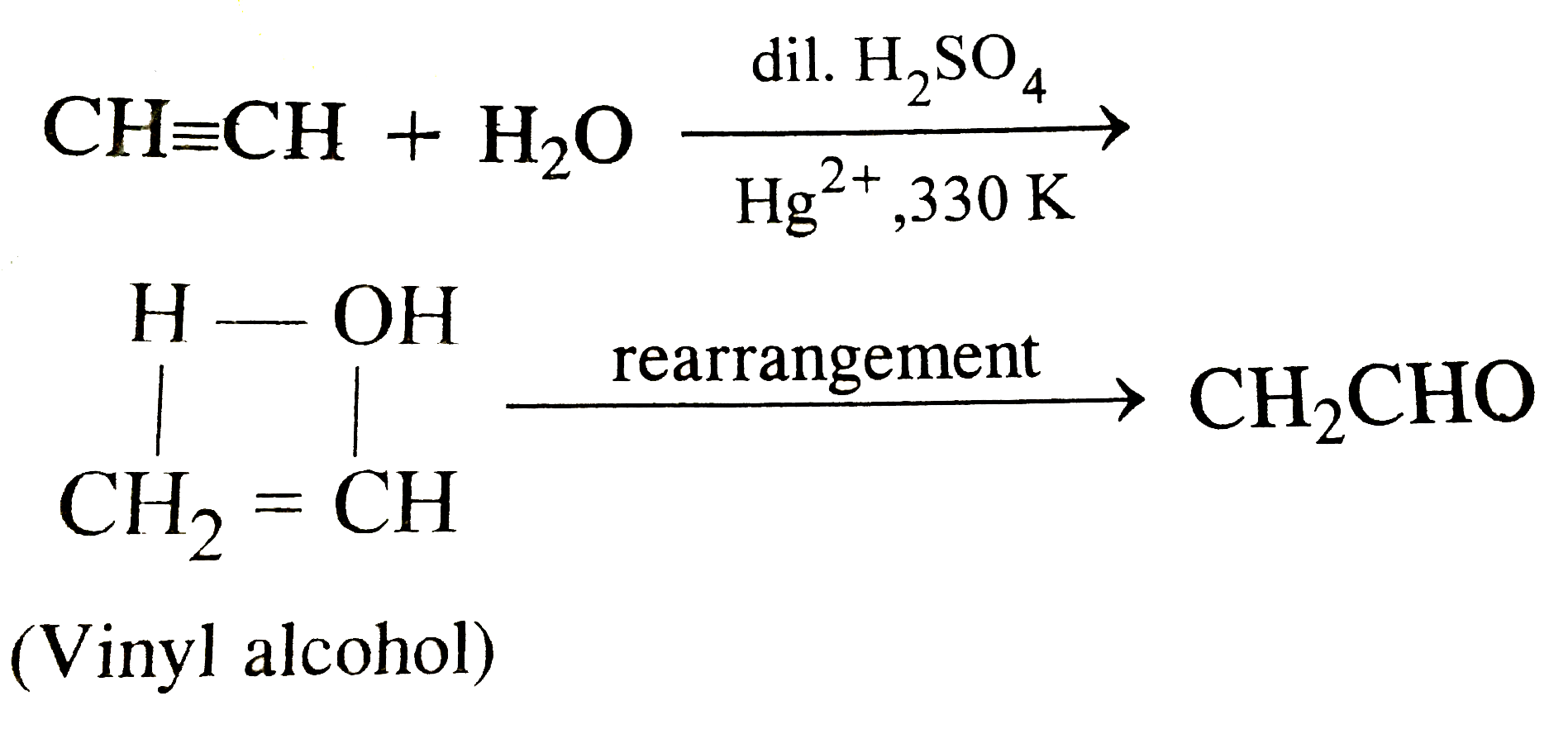Vinyl Alcohol To Acetaldehyde
Ch 3 ch o ch 2 choh h 298 g 42 7 kj mol.
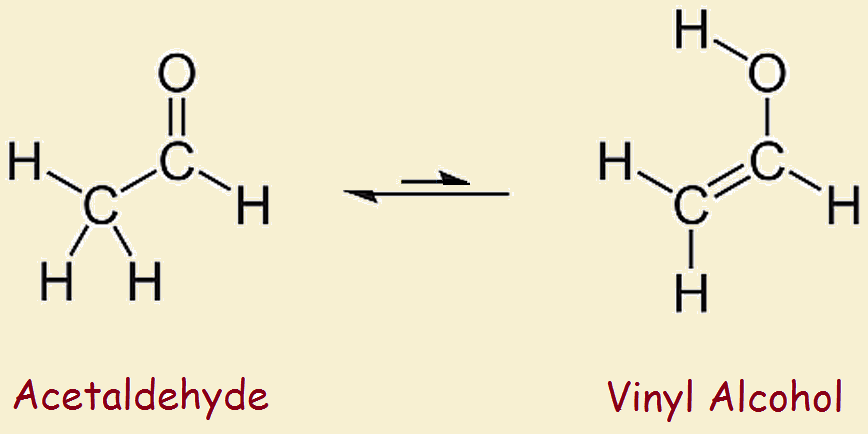
Vinyl alcohol to acetaldehyde. In vinyl alcohol there is a double bond and an alcohol that is why it is called enol form. The vinyl alcohol acetaldehyde tautomerization is an important tropospheric process. Tautomerization of vinyl alcohol. Tautomerization of vinyl alcohol to acetaldehyde.
Since vinyl alcohol is highly unstable with respect to acetaldehyde the preparation of vinyl acetate is more complex than the synthesis of other acetate esters. Under normal conditions vinyl alcohol converts tautomerizes to acetaldehyde. Determines benchmark reaction barrier heights for the catalyzed tautomerization. The atmospheric oxidation of vinyl alcohol va produced by photoisomerization of acetaldehyde aa is thought to be a source of formic acid fa.
The major industrial route involves the reaction of ethylene and acetic acid with oxygen in the presence of a palladium catalyst. In this post we wish to determine the reaction energy barrier and transition state structure for the tautomerization reaction of vinyl alcohol converting to acetaldehyde using dft. Vinyl alcohol also called ethenol iupac name is the simplest enol with the formula c h 2 choh it is a labile compound that converts to acetaldehyde it is not a precursor to polyvinyl alcohol. Atmospheric models assume implicitly that the other 86 of molecules collisionally cool reverting to thermalized acetaldehyde.
The equilibrium constant is 6 10 7 at room temperature thus that the relative amount of the enol form in a sample of acetaldehyde is very small. H 2 so 4 and hclo 4 catalysts lead to near zero reaction barrier heights. This reaction is shown below in fig 1. We demonstrate experimentally and theoretically that photo induced keto enol tautomerization of acetaldehyde to vinyl alcohol va is significant under collision free conditions.
There exists a dynamic equilibrium between the two forms. Like many other carbonyl compounds acetaldehyde tautomerizes to give an enol vinyl alcohol. Nevertheless a recent theoretical study predicted a high rate coefficient k1 298 k of 10 14 cm3 molecule 1 s 1 for the fa catalyzed tautomerization reaction 1 of va back into aa which suggests that fa buffers its own production from va. At room temperature acetaldehyde ch 3 ch o is more stable than vinyl.
Inorganic acids can efficiently catalyze the tautomerization reaction. It is not a precursor to polyvinyl alcohol. Vinyl acetate is the acetate ester of vinyl alcohol. Here we show that va is formed upon irradiation of neat acetaldehyde ch3cho in the actinic ultraviolet region between 295 and 330 nm.