Vinyl Cation Substitution Reaction

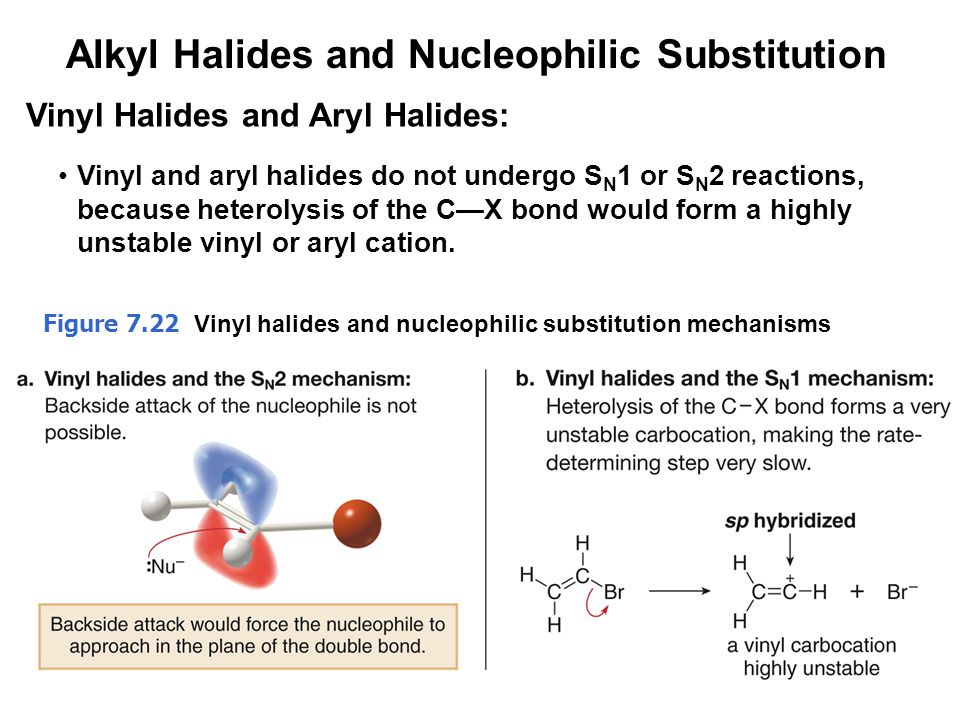
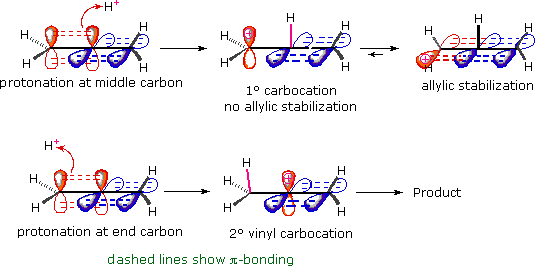
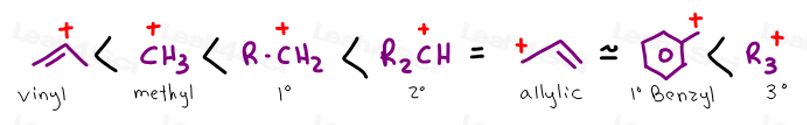
The hybridization of a vinyl carbocation is sp hybirdized.
Vinyl cation substitution reaction. An increased level of substitution at the β position of the β hydroxy α diazoketone starting material changed the course of the reaction to instead give a lactone product. Physical organic chemistry in obc. We set out to direct substitution at either side of the molecule with by using different sets of reagents. Substitution pattern of the sulfoxide on the kinetics of the reaction.
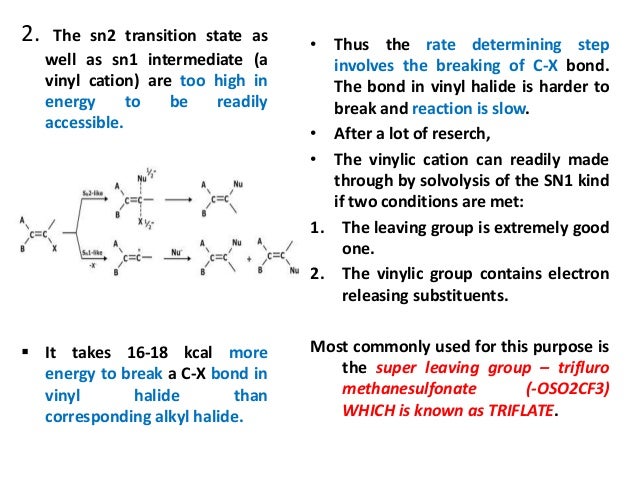
Analogous cations have proven much harder to access from vinylic carbons that are engaged in double bonds. Vinyl cations exhibit remarkable reactivity towards arene c h functionalizations. Saturated carbon centers often undergo substitution reactions by initial cleavage of a carbon halogen or carbon oxygen bond which leaves the carbon positively charged. Now show that silicon cations paired with noncoordinating anions can pull triflate groups off such vinylic.
Substituted α alkylidene cyclopentenones are formed in up to 93 yield by the intramolecular capture of vinyl cations with pendent alkenes. A reaction path that involves bond reorganization via an acylium ion. Aluminum can partially substitute for silicon in the tetrahedral t site. This computational study revealed the key mechanistic details of intramolecular c h vinylation through a vinyl cation intermediate.
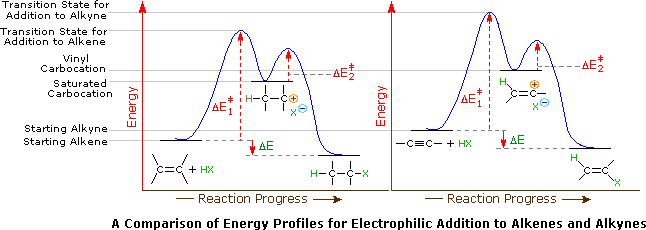
Based on the reaction mechanism the effects of substitution ring strain and tether length on mechanistic computational amp. The vinyl cation is a carbocation with the positive charge on an alkene carbon. Carbon with two other atoms attached prefers sp hybridization and a linear geometry. Reaction as shown by the formation of compounds 2k o.
Vinyl cation stabilization by silicon enables a formal metal free α arylation of alkyl ketones. Vinyl cations provides a comprehensive and detailed treatment of the reactive intermediate in which the electron deficient carbon is an integral part of a. The vinyl cations are less stable due to the difference in hybridization of the carbon bearing. Competition between vinylic substitution and conjugate addition in the reactions of vinyl selenoxides and vinyl selenones with nucleophiles in dmf.
This book emphasizes that the reaction through vinyl cations is a viable pathway among the multitude of mechanistic routes for vinylic substitution. Its empirical formula is c 2 h 3 more generally a vinylic cation is any disubstituted trivalent carbon where the carbon bearing the positive charge is part of a double bond and is sp hybridized in the chemical literature substituted vinylic cations are often referred to as vinyl cations and understood to. Between titanium and other c type cations. We first investigated the reaction between terminal propargyl silane 1 a and different sulfoxides in the presence of a brønsted acid.
A vinyl cation is a positively charged molecule a cation where the positive charge is located on a vinyl group ch ch2. Partial substitution of fluorine f chlorine and oxygen for hydroxyl oh in the hydroxyl site is also common. The complexity of the amphibole formula has given rise to numerous mineral. As shown in figure 1 when an electron rich sulfoxide such as bis p methoxyphenyl sulfoxide was used.
Vinyl carbocation is unstable.