Vinyl Chloride Thermal Cracking

Ethylene dichloride is produced through the iron iii.
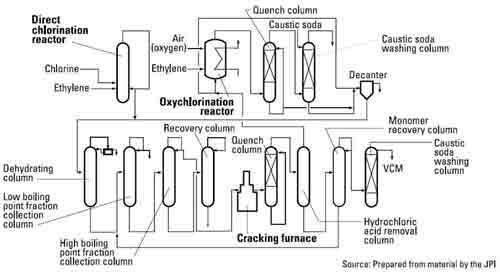
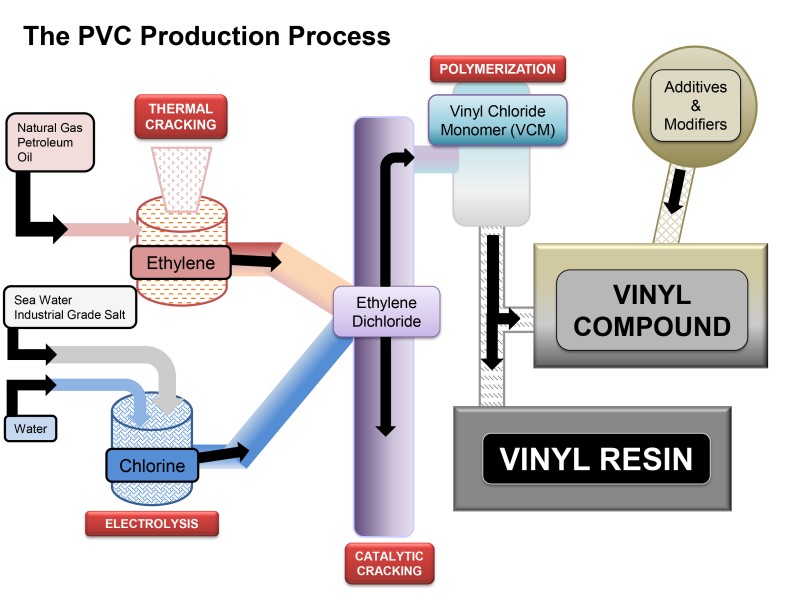
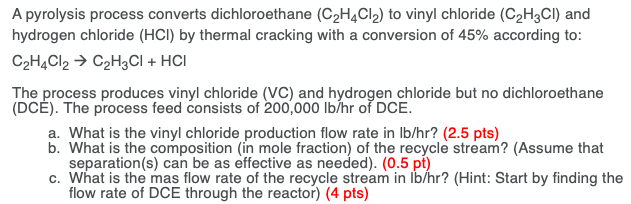
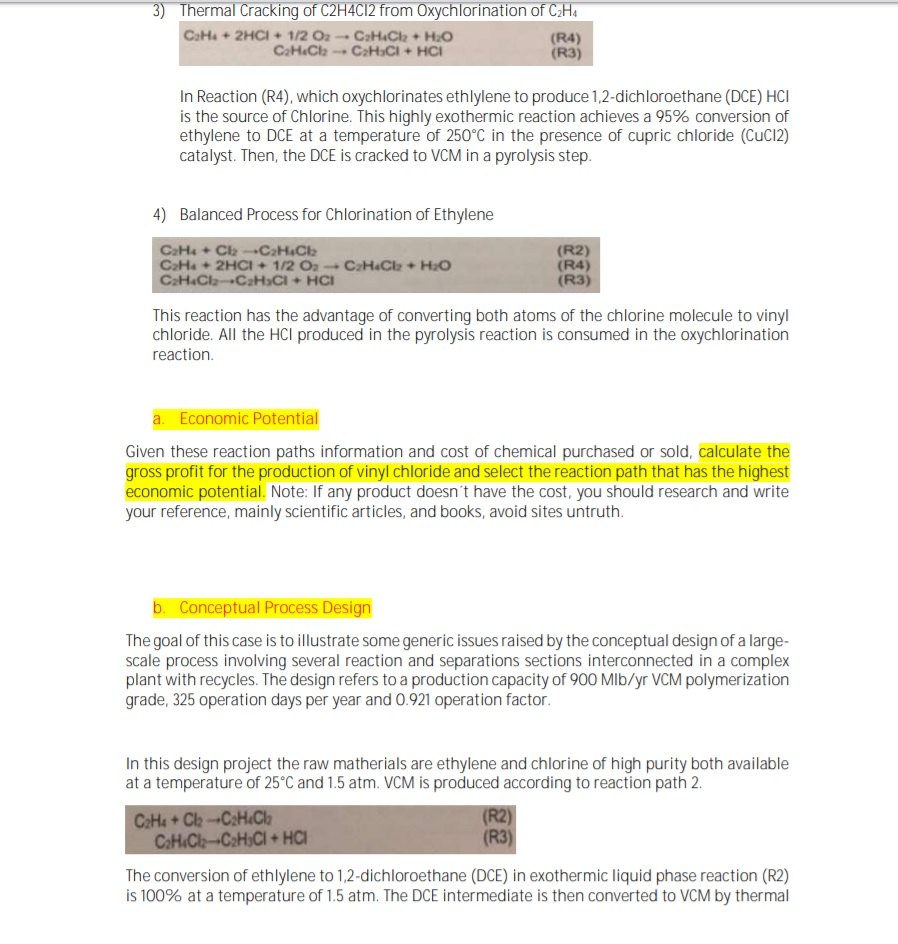
Vinyl chloride thermal cracking. Vinyl chloride monomer vcm chloroethene the major precursor for pvc production. The present invention relates to a method for production of vinyl chloride by thermal cracking of 1 2 dichloroethane edc in which it is possible to improve the energy balance time interval between sessions maintenance of the cracking furnace and or release of vinyl chloride in comparison with the modern level of technology see table 1. When heated under pressure. Pyrolysis thermal cracking of edc produces vinyl chloride.
It is the precursor to polyvinyl chloride pvc which is used. The furnace consists of four main sections. The heat required for endothermic. At high temperature ethylene dichloride decomposes into vinyl chloride monomer and hcl by a complex reaction mechanism.
Before being fed to the cracking zone the edc is brought almost to the boiling point at 15 to 31 bar and then expanded to 10 to 16 bar with flashing. The reaction mixture is directly quenched with cold edc releasing gaseous hydrogen chloride. Due to chemical reaction type and heat transfer mechanism design and simulation of cracking reactors are complicated. To this end 1 2 dichloroethane is partially evaporated in the evaporation stage at a temperature substantially.
Vinyl chloride is an organochlorine compound that is a colorless flammable gas that readily forms a liquid under increased pressure or at reduced temperatures. A radiation section a convection section a shock section and a stack. The hazards of storing and shipping acetylene meant that the vinyl chloride facility needed to be located very close to the acetylene generating facility. An improved process for the preparation of vinyl chloride from 1 2 dichloroethane edc wherein 0 10 to 0 15 by weight of carbon tetrachloride based on edc is used as a cracking promoter and the chcl 3 content is limited to less than 200 ppm.
After separation of the vinyl chloride by distillation the edc is fed back to the dehydro chlorination step. Ethylene dichloride is also used generally as an intermediate for other organic chemical compounds and as a solvent. It is a colorless liquid with a chloroform like odor. This process is very important from either chemical reaction or transport phenomena s point of view.
Production of vinyl chloride wherein 1 2 dichloroethane is evaporated under pressure in an evaporation stage and resulting 1 2 dichloroethane in vapor form is thermally cracked at a temperature substantially within the range 450 650 c with the resultant formation of vinyl chloride. When vinyl chloride producers shifted to using the thermal cracking of edc described above some used byproduct hcl in conjunction with a colocated acetylene based unit.